Etymology
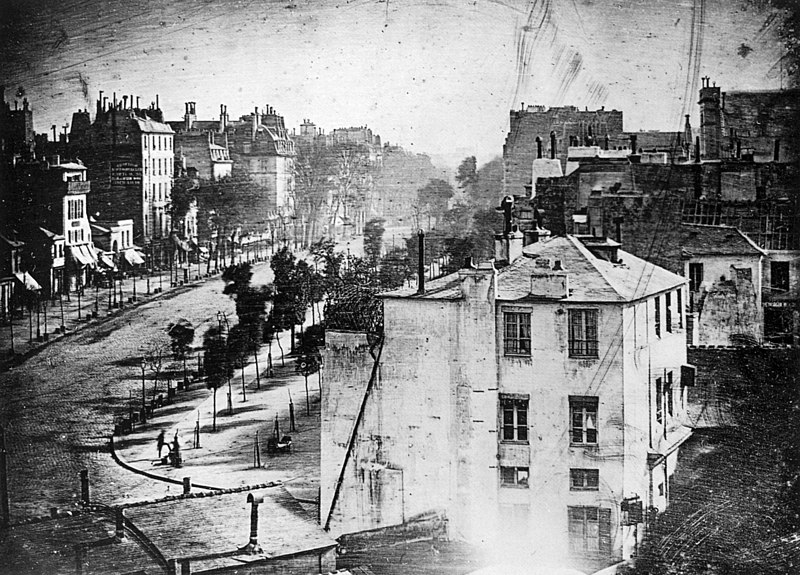
The coining of the word "Photography" has been attributed in 1839 to Sir John Herschel based on the Greek φῶς (phos), (genitive: phōtós) meaning "light", and γραφή (graphê), meaning "drawing, writing", together meaning "drawing with light"
Technological background
Photography is the result of combining several different technical discoveries. Long before the first photographs were made, Chinese philosopher Mo Ti and Greek mathematicians Aristotle and Euclid described a pinhole camera in the 5th and 4th centuries BCE. In the 6th century CE, Byzantine mathematician Anthemius of Tralles used a type of camera obscura in his experiments
Ibn al-Haytham (Alhazen) (965 in Basra – c. 1040 in Cairo) studied the camera obscura and pinhole camera, Albertus Magnus (1193/1206–80) discovered silver nitrate, and Georges Fabricius (1516–71) discovered silver chloride. Daniel Barbaro described a diaphragm in 1568. Wilhelm Homberg described how light darkened some chemicals (photochemical effect) in 1694. The novel Giphantie (by the French Tiphaigne de la Roche, 1729–74) described what could be interpreted as photography.
Early History: Development of chemical photography
Monochrome process
Around the year 1800, Thomas Wedgwood made the first known attempt to capture the image in a camera obscura by means of a light-sensitive substance. He used paper or white leather treated with silver nitrate. Although he succeeded in capturing the shadows of objects placed on the surface in direct sunlight, and even made shadow-copies of paintings on glass, it was reported in 1802 that " the images formed by means of a camera obscura have been found too faint to produce, in any moderate time, an effect upon the nitrate of silver." The shadow images eventually darkened all over because " no attempts that have been made to prevent the uncoloured part of the copy or profile from being acted upon by light have as yet been successful." Wedgwood may have prematurely abandoned these experiments because of his frail and failing health; he died aged 34 in 1805.
The oldest surviving permanent photograph of the image formed in a camera was created in 1826 or 1827 by the French inventor Joseph Nicéphore Niépce. The photograph was produced on a polished pewter plate. The light-sensitive material was a thin coating of bitumen, a naturally occurring petroleum tar, which was dissolved in white petroleum, applied to the surface of the plate and allowed to set before use. After a very long exposure in the camera (traditionally said to be eight hours, but possibly several days), the bitumen was sufficiently hardened in proportion to its exposure to light that the unhardened part could be removed with a solvent, leaving a positive image with the light regions represented by hardened bitumen and the dark regions by bare pewter. To see the image plainly, the plate had to be lit and viewed in such a way that the bare metal appeared dark and the bitumen relatively light.![One of the oldest photographic portraits known, made by Joseph Draper of New York, in 1839[8] or 1840, of his sister, Anna Katherine Draper.](http://upload.wikimedia.org/wikipedia/commons/thumb/c/ce/Dorothy_Catherine_Draper_crop.jpg/478px-Dorothy_Catherine_Draper_crop.jpg)
Niépce had previously experimented with paper coated with silver chloride. Unlike earlier experimenters with silver salts, he succeeded in photographing the images formed in a small camera, producing his first results in 1816, but like his predecessors he was unable to prevent the coating from darkening all over when exposed to light for viewing. As a result, he had become disenchanted with silver compounds and turned his attention to bitumen and other light-sensitive organic substances.
In partnership, Niépce (in Chalon-sur-Saône) and Louis Daguerre (in Paris) refined the bitumen process, substituting a more sensitive resin and a very different post-exposure treatment that yielded higher-quality and more easily viewed images. Exposure times in the camera, although somewhat reduced, were still measured in hours.
In 1833 Niépce died of a stroke, leaving his notes to Daguerre. More interested in silver-based processes than Niépce had been, Daguerre experimented with photographing camera images directly onto a silver-surfaced plate that had been fumed with iodine vapor, which reacted with the silver to form a coating of silver iodide. Exposure times were still impractically long. Then, by accident according to traditional accounts, Daguerre made the pivotal discovery that an invisibly faint latent image produced on such a plate by a much shorter exposure could be "developed" to full visibility by mercury fumes. This brought the required exposure time down to a few minutes under optimum conditions. A strong hot solution of common salt served to stabilize or fix the image by removing the remaining silver iodide. On 7 January 1839, Daguerre announced this first complete practical photographic process to the French Academy of Sciences, and the news quickly spread. At first, all details of the process were withheld and specimens were shown only to a trusted few. Arrangements were made for the French government to buy the rights in exchange for pensions for Niépce's son and Daguerre and then present it to the world (with the de facto exception of Great Britain) as a free gift. Complete instructions were published on 19 August 1839.
After reading early reports of Daguerre's invention, William Henry Fox Talbot, who had succeeded in creating stabilized photographic negatives on paper in 1835, worked on perfecting his own process. In early 1839 he acquired a key improvement, an effective fixer, from John Herschel, the astronomer, who had previously shown that hyposulfite of soda (commonly called "hypo" and now known formally as sodium thiosulfate) would dissolve silver salts. News of this solvent also reached Daguerre, who quietly substituted it for his less effective hot salt water treatment.
A calotype print showing the American photographer Frederick Langenheim (circa 1849). Note, the caption on the photo calls the process Talbotype
Talbot's early silver chloride "sensitive paper" experiments required camera exposures of an hour or more. In 1840, Talbot invented the calotype process, which, like Daguerre's process, used the principle of chemical development of a faint or invisible "latent" image to reduce the exposure time to a few minutes. Paper with a coating of silver iodide was exposed in the camera and developed into a translucent negative image. Unlike a daguerreotype, which could only be copied by rephotographing it with a camera, a calotype negative could be used to make a large number of positive prints by simple contact printing. The calotype had yet another distinction compared to other early photographic processes, in that the finished product lacked fine clarity due to its translucent paper negative. This was seen as a positive attribute for portraits because it softened the appearance of the human face. Talbot patented this process, which greatly limited its adoption. He spent the rest of his life in lawsuits defending the patent until he gave up on photography. Later George Eastman refined Talbot's process, which is the basic technology used by chemical film cameras today. Hippolyte Bayard had also developed a method of photography but delayed announcing it, and so was not recognized as its inventor.
In 1839, John Herschel made the first glass negative, but his process was difficult to reproduce. Slovene Janez Puhar invented a process for making photographs on glass in 1841; it was recognized on June 17, 1852 in Paris by the Académie Nationale Agricole, Manufacturière et Commerciale. In 1847, Nicephore Niépce's cousin, the chemist Niépce St. Victor, published his invention of a process for making glass plates with an albumen emulsion; the Langenheim brothers of Philadelphia and John Whipple and William Breed Jones of Boston also invented workable negative-on-glass processes in the mid-1840s.
In 1851 Frederick Scott Archer invented the collodion process. Photographer and children's author Lewis Carroll used this process.
Roger Fenton's assistant seated on Fenton's photographic van, Crimea, 1855.
Herbert Bowyer Berkeley experimented with his own version of collodian emulsions after Samman introduced the idea of adding dithionite to the pyrogallol developer. Berkeley discovered that with his own addition of sulfite, to absorb the sulfur dioxide given off by the chemical dithionite in the developer, that dithionite was not required in the developing process. In 1881 he published his discovery. Berkeley's formula contained pyrogallol, sulfite and citric acid. Ammonia was added just before use to make the formula alkaline. The new formula was sold by the Platinotype Company in London as Sulpho-Pyrogallol Developer.
Nineteenth-century experimentation with photographic processes frequently became proprietary. The German-born, New Orleans photographer Theodore Lilienthal successfully sought legal redress in an 1881 infringement case involving his "Lambert Process" in the Eastern District of Louisiana.






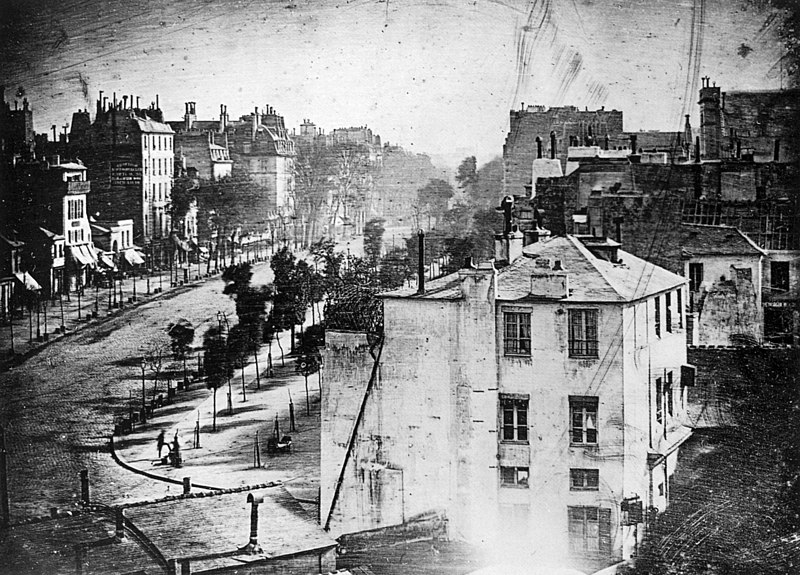
![One of the oldest photographic portraits known, made by Joseph Draper of New York, in 1839[8] or 1840, of his sister, Anna Katherine Draper.](http://upload.wikimedia.org/wikipedia/commons/thumb/c/ce/Dorothy_Catherine_Draper_crop.jpg/478px-Dorothy_Catherine_Draper_crop.jpg)










